The Biotech Revolution: Five Innovations Transforming Future Medicine
This is the first time in the 21st century that biology, engineering, and data science have worked together like this. Biotechnology was only employed in laboratory ten years ago. Now, it’s behind testing and therapies that were exclusively in science fiction. Biotechnology advancements are redefining the laws of medicine, such as carefully manipulating genes and harnessing the body’s own immune system. As we come closer to individualized, curative medicines, scientists and doctors all across the world are employing new techniques to cope with challenges that vary from uncommon genetic illnesses to worldwide pandemics.
This long article discusses about the five key achievements in biotechnology that will transform how medicine is done in the future:
- Changing Genes using CRISPR-Cas9
- Technology for mRNA vaccines
- Using CAR-T cells to treat people
- AI for Finding New Drugs
- Platforms for organoids and chips that have organs
Each part explains about the science underlying the technology, how it can be utilized in the real world, how it obtained authorization from the government, what challenges it has, and what the future holds. This book will show you how cutting-edge biology is making precision medicine possible, no matter if you’re a biotech professional, a healthcare investor, or just someone who is curious.
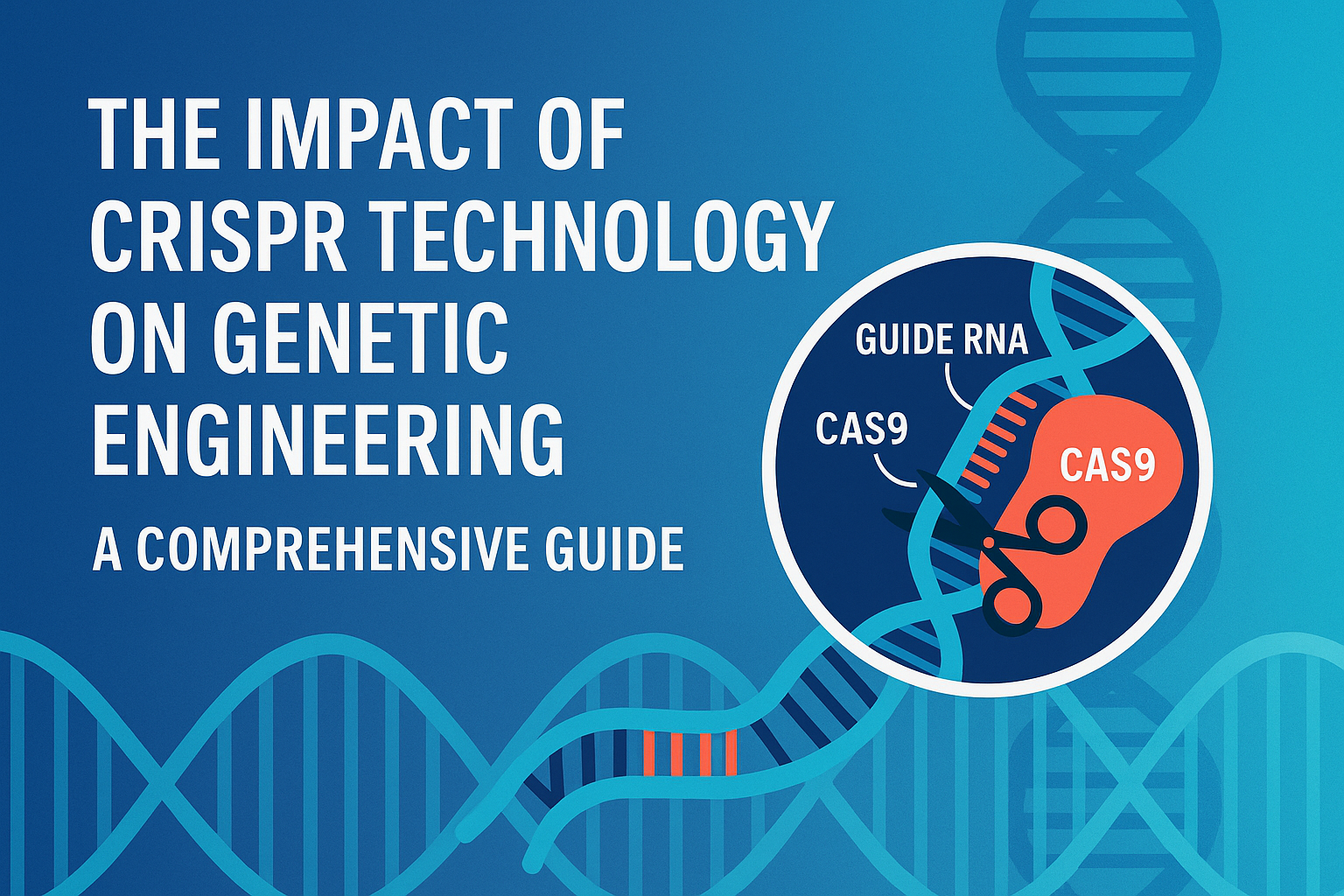
1. Changing Genes with CRISPR-Cas9
So, in brief, this is how it works:
Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) and Cas9 nuclease have made it easy for scientists to take out, add, or modify DNA sequences in living cells. The immune system of bacteria is what the CRISPR-Cas9 system is founded on. It uses a programmable guide RNA (gRNA) to discover complementary DNA, and then Cas9 cuts that DNA in two locations. After that, the cellular repair mechanisms, which are either non-homologous end joining or homology-directed repair, make the alteration happen.
Main Benefits:
- Accuracy: Base-pair specificity down to single nucleotides.
- It may be utilized with several types of cells and animals, making it flexible.
- Scalability is being able to modify more than one gene at once.
Uses in Health
How to Take Care of Blood Disorders You Get from Your Parents:
Vertex Pharmaceuticals and CRISPR Therapeutics’ CTX001 uses ex vivo CRISPR editing of patient hematopoietic stem cells to switch fetal hemoglobin back on. More than 90% of the participants who took part in multi-center Phase II trials no longer need blood transfusions.
The same platform has also shown that it can cure β-thalassemia by keeping hemoglobin levels normal for a long time.
How to take care of your eyes:
Editas Medicine and Allergan’s EDIT-101 is a therapy for Leber congenital amaurosis that works by fixing mutations in the CEP290 gene. Early Phase I/II findings reveal that the retina is working better.
Important Steps in Creating Rules
In 2024, the FDA approved CTX001 as the first CRISPR treatment. It got the Regenerative Medicine Advanced Therapy (RMAT) label and was quickly authorized for SCD. This was the first CRISPR product that the U.S. government said may be used on live beings.
On May 12, 2025, the Medicines Agency of Europe (EMA) issued ex vivo CRISPR treatment for β-thalassemia conditional approval.
Moral and practical issues
- Changes that aren’t planned could cause cancer or damage DNA, which are outcomes that aren’t what you want. These hazards are lower with better high-fidelity Cas9 variations and genome-wide screening.
- The “CRISPR babies” event in China in 2018 led to a worldwide ban on modifying the human germline. The existing agreement only lets somatic cells be used in hospitals and clinics.
- Access and Fairness: It’s impossible to be fair when therapy costs so much (more than $500,000). The point of working together with schools and businesses is to make manufacturing more efficient and assist more people acquire what they need.
What to Do Next
- Base Editing and Prime Editing are new CRISPR techniques that let you edit single nucleotides without breaking double strands. This means that additional changes can be rectified.
- Lipid nanoparticles and viral vectors (AAV) are making tissue-specific in vivo editing better, as proven in animal models for Duchenne muscular dystrophy.
- Agricultural and Environmental Uses: CRISPR is transforming the way we grow crops that can better tolerate climate change and disease.
2. What Kind of Technology Do mRNA Vaccines Use?
Plan and Rule
Messenger RNA (mRNA) vaccines use fake mRNA that is wrapped up in lipid nanoparticles and has antigenic proteins in it, mainly viral spike proteins. When injected, the mRNA is turned into protein antigens by host cells. These increase both humoral and cellular immunity without utilizing live pathogens.
A huge step forward in the fight against COVID-19
In Phase III studies, the Pfizer-BioNTech BNT162b2 and Moderna mRNA-1273 vaccines were roughly 95% successful at stopping COVID-19 from spreading and killing people around the world.
By the end of 2021, more than 2 billion mRNA dosages had been handed out all around the world. This proves that the platform is safe and can be used by a lot of people.
More Ways to Use
- In the third quarter of 2025, the Moderna mRNA-1010 multivalent flu vaccine will move on to Phase III. It will target a variety of distinct types of the flu (Moderna Pipeline).
- In studies for melanoma and glioblastoma, BioNTech’s tailored neoantigen vaccines exploit mutations that are unique to tumors to create immune responses that endure a long time.
- Translate Bio and other companies are looking into mRNA as a way to provide therapeutic proteins to people with uncommon genetic disorders like cystic fibrosis.
Better ways to make and share stuff
- Cell-Free Synthesis: You can produce mRNA in vitro, which means you don’t need as many cells to develop and you can do it faster.
- At 2–8 °C, freeze-dried formulations survive longer, which makes it easier to carry them around in regions with low resources.
The rules and safety environment
- Safety Profile: mRNA doesn’t get into the genome, and the risk goes down over time because it doesn’t last long. Most adverse effects, such as a minor fever and soreness at the injection site, usually away after a few days.
- The FDA fully approved BNT162b2 (Comirnaty) on August 23, 2021. Then, the EUA was extended for kids. Before COVAX could send out the vaccines, the EMA and WHO gave its approval.
What’s Next
- Self-amplifying mRNA (saRNA) has a replicase gene that allows it reproduce itself inside cells. This could mean that you don’t require as much of it (Molecular Therapy, 2023).
- Universal mRNA platforms for pan-coronavirus vaccines attempt to target conserved epitopes, which will stop future pandemics.
- New ways to give medications without viruses: Polymer nanoparticles and exosomes may make it even easier to reach to the proper tissues.
3. Therapy with CAR-T Cells
The Science That Explains It
Chimeric Antigen Receptor (CAR) T-cell treatment modifies a person’s own T lymphocytes by giving them false receptors that can locate antigens that are only on malignancies. The modified CAR-T cells proliferate in the body and assault cancer cells with robust reactions that kill cells.
Important Approvals
- The FDA gave Kymriah (tisagenlecleucel) the green light in 2017 as the first CAR-T for juvenile acute lymphoblastic leukemia (ALL) (FDA Prescribing Information).
- Yescarta (axicabtagene ciloleucel) was licensed for use in people with large B-cell lymphoma in 2017.
- The RMAT approved Breyanzi (lisocabtagene maraleucel) for diffuse large B-cell lymphoma in 2021.
Effects on Health
Cancers of the blood:
- More than 80% of persons with refractory ALL go into complete remission, while about 50% of people with aggressive lymphomas do.
- Durability: Data from follow-up suggests that the median overall survival for responders is more than 24 months.
Problems with solid tumors:
Tumor microenvironment immunosuppression, antigen heterogeneity, and physical barriers are some of the problems that solid tumors cause. HER2 (glioblastoma) and GPC3 (liver cancer) are currently being tested, but they need to be halted because they hurt healthy cells that are not in the tumor.
New ways to do things
- Automated Bioreactors: Closed-system platforms lower the risk of contamination and the quantity of work that needs to be done.
- Allogeneic “Off-the-Shelf” CAR-T: Allogene Therapeutics and other companies employ gene editing to generate T cells that can be given to anyone. This means they don’t have to make them for each patient.
What to Do When Things Go Wrong
- Cytokine Release Syndrome (CRS) makes you have a fever, low blood pressure, and difficulties with your organs. Blocking the IL-6 receptor with tocilizumab is the first step in treatment.
- Corticosteroids and other forms of support are used to treat seizures and encephalopathy that are part of Immune Effector Cell-Associated Neurotoxicity Syndrome (ICANS).
Next-Gen CARs
- Multi-Antigen CARs: Tandem CARs aim at two antigens to stop them from escaping.
- Logic-Gated CARs: Preclinical models of pancreatic cancer reveal that “AND/OR/NOT” Boolean circuits make tumors more selective (Cell, 2024).
- Safety Switches: Inducible caspase-9 systems help you safely get rid of CAR-T cells if they become too toxic.
4. Using AI to Find New Medications
The Rise of Computational Biology
Artificial intelligence (AI) and machine learning (ML) are speeding up the hunt for novel pharmaceuticals by leveraging large datasets including genomic, proteomic, and clinical data to forecast how drugs will work with their targets, improve existing drugs, and uncover new uses for old drugs.
Great Achievements
- AlphaFold2 from DeepMind is an AI that folds proteins. It was able to hit 98.5% of its targets with virtually atomic accuracy. This revolutionized how medications are made based on their structure.
- Baricitinib Repurposing by BenevolentAI: ML models showed that baricitinib, a JAK inhibitor, may be utilized to treat COVID-19, and an EUA was given within months.
Platforms and Partnerships
- Using generative adversarial networks, Insilico Medicine produces new tiny compounds. Two applicants moved on to Phase I in 2023.
- Exscientia announced that DSP-1181, the first medicine made by AI, would begin clinical trials in 2020 for persons with obsessive-compulsive disorder.
Merging workflows
- Finding Targets: ML classifiers use genomic and phenotypic screening data to choose which targets to focus on.
- Lead Optimization: Generative models suggest novel scaffolds, and reinforcement learning improves binding affinity and ADME/Tox profiles.
- Preclinical Validation: Predictions derived in silico speed up testing in vitro and in vivo, which implies fewer failures.
Things to remember about IP and rules
- The FDA and EMA, two groups that make rules, are giving recommendations on AI software as a medical device (SaMD) and how it may help make pharmaceuticals.
- When AI designs make first-in-class compounds, patent offices are looking at what makes something patentable again.
The Future’s Horizons
- Digital twins are virtual duplicates of patients that integrate data from several omics and lifestyle sources to assist doctors choose the optimum medications and doses for each patient.
- Quantum Computing: New quantum algorithms believe they can run molecular simulations that traditional computers couldn’t handle.
5. Organoids and Organs on a Chip Platforms
A Group of Ideas
Organoids are small organs created from stem cells that resemble and act like genuine tissues. They are three-dimensional and can put themselves in order. Microfluidics and living cells are used in organ-on-a-chip systems to produce microchips that look like the regions where genuine organs live.
Uses in Making Drugs
Making models of illnesses:
- We evaluate CFTR modulators like ivacaftor on the epithelium of cystic fibrosis patients using intestinal organoids to determine the optimum medication for each person.
- Brain Organoids: Learn about how the Zika virus affects neurons and how Alzheimer’s disease works in 3D networks of neurons.
- Toxicity Testing: Heart-on-a-chip platforms that use cardiomyocytes from iPSCs can detect heart issues that are toxic sooner than animal models.
- Precision Oncology: Tumor organoids produced from patient biopsies can tell you how well chemotherapy will work more than 80% of the time. This helps doctors figure out the best strategy to treat malignancies of the pancreas and colon.
Business and rules are moving ahead.
- The FDA is looking at Emulate Inc.’s human emulation systems as places to test pharmaceuticals before they are put on the market to check if they are safe.
- Hubrecht Organoid Technology (HUB) uses quality management methods to give academic and industry partners biobanked human organoids.
Problems with technology
- Cells don’t have blood vessels or immune cells, so it’s challenging to maintain them alive for a long time and fully simulate how they would respond in the body.
- Standardization: It’s challenging to acquire the same findings because each lab does things in its own way. In 2024, the EURO‑CARDIOCHIP group wants to develop rules that everyone agrees on.
Things You Should Know
- Multi-Organ Chips: These chips come from the liver, kidneys, and stomach and are linked together to show how medications are broken down and how organs communicate with each other.
- Using AI, high-content imagery, and machine learning combined to automatically figure out phenotypes and make predictions.
Final Thoughts
Biotechnology is no longer a modest field; it is now the most important aspect of medicine today. With CRISPR gene editing, the genome is being changed with an accuracy that has never been seen before. Vaccines are created differently now that mRNA vaccines are available. During the COVID-19 pandemic, they were quite significant, and they will be vital for immunotherapies in the future. One fantastic example of how tailored immuno-oncology can assist treat blood malignancies is CAR-T cell therapy. AI-driven medication discovery uses data to find new uses for old pharmaceuticals and make new ones faster. Organoids and organ-on-a-chip platforms generate models that are more like people, so we don’t have to test on animals as much. This makes the results more correct.
These innovations mark the start of a new era in medicine that is personalized, accurate, and focused on preventing illness. They are founded on careful study and tight guidelines. But we still have to work together on some challenges, like moral ones, expensive expenses, and technical ones. As we move forward, professionals in academia, business, and government will need to work together across professions to make sure that these new ideas lead to fair healthcare outcomes.
Questions and Answers That Are Common
Q1: What is the most important safety issue with CRISPR-Cas9 treatments?
Answer: Changes that happen without your permission and don’t happen where you want them to can turn on oncogenes or screw up tumor suppressor genes. High-fidelity Cas9 variations and genome-wide off-target screening techniques make this danger reduced.
Q2: What does mRNA vaccines apart from other types of vaccines?
Answer: Traditional immunizations use dead germs or protein pieces, whereas mRNA vaccines educate cells how to create the antigen themselves. You can swiftly create and make things on a huge scale with this platform without needing to cultivate live viruses.
Q3: Is it possible to employ CAR-T on solid tumors?
Answer: Scientists are undertaking early studies, but solid tumors are tricky to treat because they have various antigens, physical obstacles, and surroundings that make the immune system less effective. Next-generation CAR designs, such as logic gates and multi-antigen targeting, as well as combination therapies, are being employed to get past these difficulties.
Q4: How good is AI at finding out which new medications will work?
Answer: AI can put targets in order of importance and offer candidate chemicals that are more likely to hit than random screening. For example, Exscientia’s AI-designed DSP-1181 passed preclinical testing that looked at its binding and ADME profiles before going through clinical trials.
Q5: What are organoids, and why do they matter?
Answer: Organoids are small pieces of tissue generated from stem cells that put themselves together and work like real organs. They help us learn more about diseases and test medications by filling up the space between 2D cell cultures and animal models.
References
- ClinicalTrials.gov. A Study of CTX001 in Subjects With Severe Sickle Cell Disease. Retrieved July 28, 2025, from https://www.clinicaltrials.gov/ct2/show/NCT03655678
- Frangoul, H. et al. (2020). CRISPR‑Cas9 Gene Editing for Sickle Cell Disease and β‑Thalassemia. Nature, 595(7867), 538–543. https://www.nature.com/articles/s41586-020-2536-9
- Lima, B. et al. (2021). In Vivo CRISPR Gene Editing in Light‑Sensitive Retinal Cells. Science Translational Medicine, 13(578), eabc1774. https://stm.sciencemag.org/content/13/578/eabc1774
- Polack, F. P. et al. (2020). Safety and Efficacy of the BNT162b2 mRNA COVID‑19 Vaccine. New England Journal of Medicine, 383(27), 2603–2615. https://www.nejm.org/doi/full/10.1056/NEJMoa2034577
- Vogel, A. B. et al. (2022). Self‑Amplifying mRNA Vaccines: A New Frontier. Molecular Therapy, 30(3), 1032–1047. https://www.cell.com/molecular-therapy-family
- Maude, S. L. et al. (2019). Tisagenlecleucel in Children and Young Adults with B‑Cell Lymphoblastic Leukemia. New England Journal of Medicine, 380(5), 488–498. https://www.nejm.org/doi/full/10.1056/NEJMoa1815703
- AlphaFold Team. (2021). Highly Accurate Protein Structure Prediction with AlphaFold. Nature, 596(7873), 583–589. https://www.nature.com/articles/s41586-021-03819-2
- Richardson, P. et al. (2020). Baricitinib as Potential Treatment for COVID‑19. Nature Biotechnology, 38(11), 1347–1355. https://www.nature.com/articles/s41587-020-0649-5
- Sato, T. et al. (2018). Single Lgr5 Stem Cells Build Crypt–Villus Structures In Vitro. Cell Stem Cell, 22(2), 184–197. https://www.cell.com/cell-stem-cell/fulltext/S1934-5909(18)30318-4
- Huh, D. et al. (2013). Reconstituting Organ‑Level Lung Functions on a Chip. Science, 328(5986), 1662–1668. https://www.science.org/doi/10.1126/science.1231294